
During formation of the neuromuscular junction (NMJ) in Drosophila melanogaster, Wnt signaling mediates maturation of the synapse by promoting mRNP export from muscle cell nuclei ( Speese et al., 2012). Recent work from the Budnik group suggests that nuclear export by NE budding might also be important for normal development ( Speese et al., 2012). NE budding as an alternate mechanism for nuclear export Thus, accumulating evidence suggests that much remains to be learned about the NE barrier and its remodeling during interphase in normal and diseased cells. Fourth, two kinds of NE fusion have been described (1) the ONM and INM fuse to make a channel through the NE to accommodate NPC insertion, and (2) the ONM and then INM of two separate nuclei fuse to make one contiguous nucleus (see Fig. However, the membrane does not repair, and instead ER tubules mislocalize to the chromatin (see Fig. Third, NE collapse is similar to NE rupturing in that both involve a rapid loss of nuclear integrity associated with lamina gaps and chromatin herniation. Second, transient NE rupturing is characterized by a sudden loss of compartmentalization, causing mislocalization of both nuclear and cytoplasmic components, followed by the restoration of NE integrity without cell death (see Fig. Lamina disruption is required for budding. In this process, INM-derived vesicles bud into the perinuclear space and fuse with the ONM to release enclosed nuclear contents into the cytoplasm with no obvious loss of nuclear integrity or cell viability. First, NE budding has been identified as an export mechanism for large nuclear particles (see Fig. At this time, four main types of nonmitotic NE remodeling have been characterized, and will be the focus of this review. Unexpectedly, however, it has been shown that the NE can also undergo extensive remodeling in interphase, despite the importance of nuclear compartmentalization for eukaryotic cell biology. NE breakdown during mitosis has been the focus of many studies and is a dramatic example of endomembrane reorganization ( Güttinger et al., 2009). The expression of lamin and lamin-associated proteins varies widely between cell types, likely due to different requirements for nuclear mechanical stiffness and chromatin organization in cells with different functions ( Burke and Stewart, 2013). The lamin proteins interact with transmembrane INM proteins, like LBR and Lap2, and chromatin-binding proteins, like BAF, at the nuclear periphery to form a stable network that supports the membrane and links the INM to the chromatin ( Ellenberg et al., 1997 Moir et al., 2000 Wilson and Foisner, 2010). The two major types of lamin proteins are the B-type, lamins B1 and B2, and the A-type, lamins A and C, which are different isoforms of the same gene ( Dechat et al., 2010).
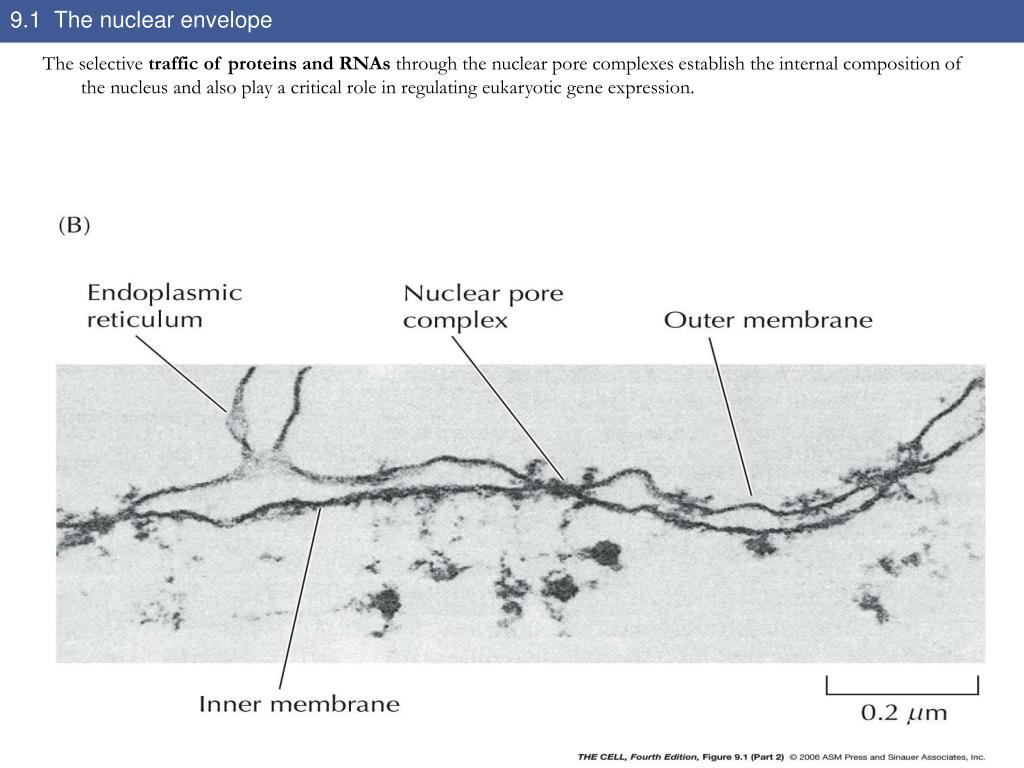
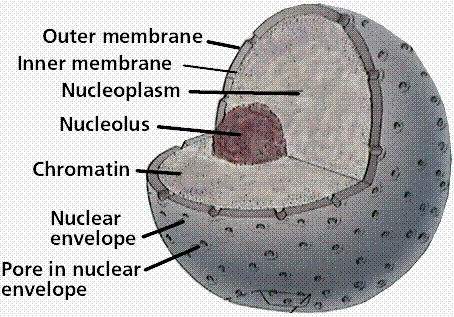
The nuclear lamina is a dense meshwork of lamin filaments attached to the INM. The NPCs extend through both the INM and ONM as well as the lamina ( Schermelleh et al., 2008) and regulate the passage of macromolecules with molecular weights exceeding ∼40 kD between the nucleus and the cytoplasm ( Wente and Rout, 2010). The nuclear membrane covers the chromatin and restricts nuclear–cytoplasmic trafficking to the NPCs. The nuclear membrane is divided into the inner nuclear membrane (INM) and outer nuclear membrane (ONM) based on protein content, but the membranes are contiguous with each other and with the ER. The nuclear envelope (NE) in animal cells comprises three structures: the nuclear membrane, the nuclear pore complex (NPC), and the lamina.


 0 kommentar(er)
0 kommentar(er)
